Pharmaceuticals
Environmental Test Chambers for the Pharmaceuticals Industry
Verify the integrity of your product's reliability throughout its shelf life.
The pharmaceutical sector heavily relies on environmental test chambers throughout various stages, including research, development, and manufacturing. They conduct stability and stress tests to determine licensed drug expiration dates and how they function during temperature changes and exposure to ultraviolet rays.
This industry is dedicated to developing, producing, and distributing licensed drugs, vaccines, blood products, biologics, and more. Ensured that pharmaceutical quality adhered to stringent standards, notably by the US Food and Drug Administration (FDA). Compliance with these regulations mandates the establishment of robust quality management systems and the maintenance of dependable testing laboratories.
AES specializes in designing, manufacturing, installing, and supporting standard and customized environmental test chambers tailored to test temperature and humidity variations within the pharmaceutical industry.
Test Chambers for Pharmaceuticals: Talk to an Expert
RELEVANT ENVIRONMENTAL TEST CHAMBERS
Stability Chambers
Conduct temperature and humidity cycling and steady-state testing.
Double stack options are available (for the 1 cu ft model).
Workspace Range: 1, 5, and 10 cu ft.
Temperature Only Chambers
Conduct testing for precise temperature control and uniformity —benchtop, stackable, and walk-in room options.
Workspace Range: 2 to 264 cu ft
Browse Temperature Only Chambers >
Temperature & Humidity Chambers
Conduct temperature and humidity cycling testing. Benchtop, stackable, and walk-in room options
Workspace Range: 2 to 264 cu ft
Browse Temperature & Humidity Chambers >
Lab Ovens
Lab ovens are used for heating and drying. We also offer liquid-cooled ovens that can reach even more extreme temperatures.
Workspace Range: .69 cu ft to 96 cu ft.
Browse Lab Ovens >
Salt Spray Chamber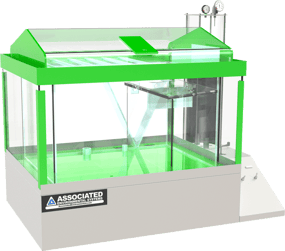
Creates a corrosive environment without introducing other contaminants into the test area.
Workspace Range: 2 to 264 cu ft
Clients




-1.png?width=277&height=184&name=SD-508%20(2)-1.png)